Abstract
To better understand the nature of acute GVHD in children, their response to upfront steroid therapy, and to test whether our MN acute GVHD risk score which was developed primarily in adult patients ( BBMT , 2015;21:761) demarcates risk in children, we examined the acute GVHD clinical stage, grade and response to prednisone 60 mg/m2 of 370 pediatric patients (median age 7 years, range 0.2-17.95 years) treated at the University of Minnesota (MN) from 1990 to 2016. Underlying diseases included malignancies (53%), inborn errors of metabolism (25%), and other non-malignant diseases (22%). Graft sources included HLA-identical sibling bone marrow or peripheral blood (BM/PB) (n=61), mismatched related BM/PB (n=8), matched unrelated BM/PB (n=104), mismatched unrelated BM/PB (n=65) single umbilical cord blood (UCB; n=97) or double UCB (n=35). The majority (95%) received myeloablative conditioning regimens.
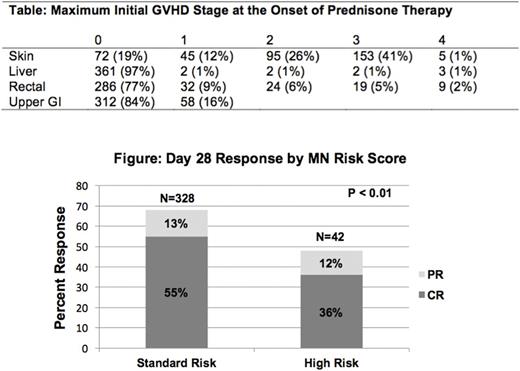
Initial GVHD organ involvement was skin only (n=250; 68%), upper and/or lower GI only (n=67;18%), liver only (n=3;1%) or multi-organ (n=50;14%) The maximum initial GVHD stage at the onset of prednisone therapy is shown in the Table. Initially, 307 (83%) patients had grade I-II GVHD, 63 (17%) had severe grade III-IV GVHD, 328 (89%) had MN standard risk GVHD and 42 (11%) had MN high risk GVHD.
Overall response (CR+PR) at day 28 was observed in 65% patients with CR in 52% and PR in 13%. Initial GVHD grade did not predict overall response at day 28 (68% for grade I, 67% for grade II, 59% for grade III, and 43% for grade IV, p=0.21). However, as shown in the Figure, MN risk score significantly demarcated day 28 response with 68% standard risk patients achieving CR/PR at day 28 compared to only 48% high risk patients (p<0.01).
In multiple regression analysis, the odds of day 28 CR/PR was lower in patients with MN high risk GVHD (Odds ratio [OR] 0.4, 95% CI 0.2-0.8, p=0.01) and in recipients of HLA mismatched unrelated donor grafts (OR 0.3, 95% CI 0.2-0.7, p=0.01). Patients without CR/PR at day 28 had higher transplant related mortality (TRM) at 2 years (56%, 95% CI 46-66%) than patients with CR (25%, 95% CI, 19-32%) or PR at day 28 (23%, 95% CI, 11-35%, p<0.01). Patients with MN high risk GVHD had a 1.6-fold increased risk of TRM (cumulative incidence 47%: (hazard ratio 1.6, 95% CI, 1.0-2.7, p=0.05) compared to MN standard risk acute GVHD (35% 2 year TRM). Risks of TRM were also significantly higher in older patients, recipients of HLA-partial and mismatched adult volunteer URD grafts (but not umbilical cord blood), and in those with non-malignant diseases.
When compared to our previously published series of predominantly adult patients, these data demonstrate that pediatric patients with acute GVHD more often have isolated skin involvement, less liver involvement and less multi-organ involvement than adults, yet a similar proportion have MN standard versus high risk GVHD. Importantly, pediatric patients respond to upfront steroid GVHD therapy at a similar frequency as adult patients. It is imperative that children be included in future trials to investigate less toxic therapies for standard risk patients and novel approaches for those with high risk GVHD. As in the adult population, compared to initial GVHD grade, the MN GVHD risk score better demarcates risk of steroid failure and TRM in pediatric patients and should be used for risk stratification in future clinical GVHD trials.
MacMillan: Magenta Therapeutics: Research Funding. Holtan: Incyte: Other: One-time advisory board member.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal